Stem Cell Therapy
Bodies heal through tissue regeneration. Damaged cells are replaced by new cells that our body naturally generates. Sufficient nutrients, natural growth factors, and stem cells must be present for regeneration to occur effectively. Collagenous tissue in joints, however, can be slow to heal, and healing is often incomplete. This is largely due to blood flow needed to supply cells necessary for regeneration. The thick, fibrous tissue in joints is great for stability, but inhibits blood flow to supply what the body needs to heal.
Another reason that adequate healing may not occur is that stem cells and nutrients in surrounding tissue may have become depleted due to chronic or severe injury, arthritis, or poor cellular health. When availability of stem cells and necessary healing cells does not keep up with healing demands, the body needs a little help.
Regenerative Orthopedic procedures, such as Bone Marrow Aspirate Concentrate commonly known as Stem Cell Therapy, are often referred to as “orthobiologics.” They use naturally derived proteins, growth factors, and stem cells to stimulate and enhance your body’s healing of muscles, bones, tendons, ligaments, and joints. The quarterback on this team of healing cells is mesenchymal stem cells.
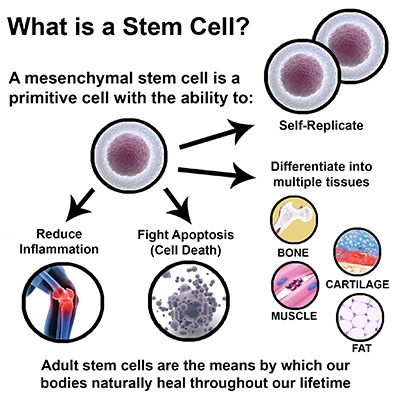
What are stem cells?
The presence of stem cells is key in stimulating the body's natural repair system by inducing the proliferation of cells. Research that examines the biological mechanisms of cellular health and tissue regeneration is extensive, particularly the role of platelets, stem cells and natural growth factors in the blood and bone marrow. Stem cells are present in all of us, acting as a repair system for the body. Mesenchymal stem cells (MSCs) have two primary functions: differentiation into functional cells such as cartilage and coordination of the healing process.
How does Stem Cell Therapy work?
Stem Cell Therapy is a non-invasive treatment designed to help the body repair damaged cells. With increased age or chronic injury, often the optimal amount of stem cells is not available. Injury, arthritis and poor cellular health disrupt the availability of stem cells needed for tissue repair resulting in the body's inability to keep pace with healing requirements. When this demand outpaces the supply, the body needs a little help. The potential to treat conditions, such as osteoarthritis, provides patients with alternatives to invasive surgery. Bone Marrow Stem Cell Therapy has been shown to improve joint function and reduce pain and inflammation. Read more about how this works.
Bone marrow derived stem cells
- Bone marrow contains nutrient rich cells to enhance healing process.
- Mesenchymal stem cells are able to differentiate into the type of cell in their surrounding environment to replace damaged ones.
- MSCs also coordinate healing through a process called "signaling." Cell signaling is the body's way of communicating to coordinate our innate repair system.
- MSCs regulate the immune system and healing response by attracting cells to areas where they are most needed.
Leader in Orthobiologic Treatment for Musculoskeletal Conditions
Dr. Minotti began performing Orthobiologic Therapy in 2007 and has treated hundreds of patients with amazing success. 86% of his patients have found significant relief from pain and restoration of musculoskeletal function.
Dr. Minotti is specialty trained in Neuromusculoskeletal Medicine. He combines his intensive training in the musculoskeletal system with advanced training in Regenerative Medicine, specifically as applied to orthopedic conditions. He conferences regularly with the world's leading physicians to provide his patients with the most effective treatments that science has to offer.
Founder of North Texas Musculoskeletal Medicine in Southlake TX, Dr. Minotti believes that orthobiologic procedures, such as Platelet Rich Plasma (PRP) and Stem Cell Therapy, are a new frontier in medicine, offering treatments that effectively bridge the gap between medications that mask pain and invasive surgery. He has helped thousands of patients find freedom from pain to restore their quality of life. Dr. Minotti is honored to be recognized by his peers with the Top Docs award. He also serves as an adjunct professor at University of North Texas Health Science Center.

Regenexx is a world-wide network of select, highly skilled physicians who are specially trained in interventional procedures for orthopedic conditions. Regenexx provides the world's most advanced, research-driven, regenerative orthopedic procedures in North America, Europe, the Middle East, Asia and Australia. Specializing in advanced non-surgical procedures for joint Injuries, osteoarthritis, and other orthopedic conditions, Dr. Minotti brings these procedures to you!
If you have an injury, or if you have tendons or ligaments that have become inflamed, stem cell therapy may help. It uses your body's own stem cells to help heal damage. It may help you avoid surgery.
To determine if Regenerative Orthopedics will be helpful for you, please tell us about your condition.
Common Applications
Numerous conditions can be considered for PRP. Based on current research and clinical experience, moderate to severe cases of osteoarthritis and tendon injuries show promising results.
- Knee Pain – Osteoarthritis, Meniscus Tears, Chondromalacia Patella, Tendon Injuries, Ligament sprains or tears.
- Hip Pain – Osteoarthritis, Hip Labrum Tears, SI Joint Arthritis, Gluteal tendon tears, Iliotibial Band (ITB) Syndrome
- Shoulder – Osteoarthritis, Rotator Cuff Tendinitis, Tendinopathy, or Tears, Labrum Tear, Bicipital Tendinitis
- Elbow Pain – Lateral Epicondylitis (Tennis Elbow), Medial Epicondylitis (Golfers Elbow), Osteoarthritis
- Wrist/Hand Pain – Osteoarthritis, DeQuervain's Tenosynovitis
- Ankle & Foot Pain – Achilles Tendinitis or Partial Tears, Plantar Fasciitis Ankle sprains/ligament injury
 Procedure Snapshot
Procedure Snapshot
First, a local anesthetic is applied to numb the area of extraction. The bone marrow is extracted from the back of the patient's pelvis or hip bone using a special needle developed for bone marrow extraction. The collected marrow is filtered and spun in a centrifuge to separate the platelets and stem cells to produce the bone marrow aspirate concentrate (BMAC).
The cells are not altered with additives or manipulated. The BMAC is then injected back into the patient's site of injury guided by ultrasound where it can begin its accelerated regenerative healing. This same-day treatment takes about 1-2 hours. Typically, 1 treatment, often with platelet-rich plasma given at follow-up, is sufficient.
Scientific advances in the understanding of how the body actually heals through tissue regeneration, combined with rapid developments in stem cell biology, have truly created a renaissance in medical treatment. Stem cells, like other medical products that are intended to treat, cure or prevent disease, require U.S. Food and Drug Administration (FDA) approval before they can be marketed. At this time, only cells harvested from the patient's own body and that are only minimally manipulated have been approved by the FDA.
Additionally, devices that allow physicians to minimally manipulate blood, bone marrow and adipose tissue for the treatment of orthopedic conditions also require FDA approval. NTXMSK recognizes and welcomes the FDA's important role in assuring patient safety. All of our treatment protocols are compliant with the published FDA guidelines regarding the use of human cells, tissues, and cellular or tissue-based products.
To learn more about what we can do to help with your condition, call our office at 817-416-0970. We will thoroughly diagnose your condition and present you with treatment options. From there we will guide you along your road to recovery.